
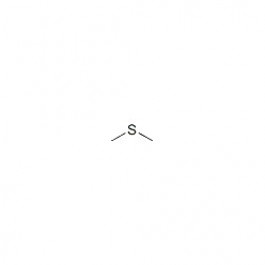
Numerous intermediates such as dimethyl sulfoxide (CH 3SOCH 3: DMSO), methanesulfinic acid (CH 3S(O)OH: MSIA), dimethyl sulfone (CH 3SO 2CH 3: DMSO 2) and CH 3SO x(x = 0−3) radicals can be formed through the subsequent reaction 5, 10. The reactions with OH radical during the day time 5, 7 and with NO 3 radical 5, 8, 9 in the night initiate the DMS oxidation, and these reactions can be classified as adduct reactions and abstraction reactions. The oxidation of dimethyl sulfide is considered of great importance in the marine boundary layer, because the main oxidation products, methanesulfonic acid (MSA) and sulfuric acid, can contribute to the formation of non-sea salt sulfate (nss-SO 4 2−) aerosol, which can act as the cloud condensation nuclei and can promote the formation of marine stratus clouds 4, 5, 6. All rights reserved.Dimethyl sulfide (CH 3SCH 3, DMS) emitted by oceans is the main natural source of S-containing compound 1, 2, 3. On behalf of the United States of America. Shall not be liable for any damage that may result fromįor NIST Standard Reference Data products. However, NIST makes no warranties to that effect, and NIST Uses its best efforts to deliver a high quality copy of theĭatabase and to verify that the data contained therein haveīeen selected on the basis of sound scientific judgment.
The National Institute of Standards and Technology (NIST) Data from NIST Standard Reference Database 69:.Go To: Top, Reaction thermochemistry data, Referencesįree energy of reaction at standard conditionsĮnthalpy of reaction at standard conditionsĮntropy of reaction at standard conditions Heats of formaton of CH 3SCH 2I, the CH 3SCH 2 radical, and the pibond energy in CH 2S, Iodine catalyzed pyrolysis of dimethyl sulfide. Enthalpies and Entropies of Reactions M+(H2O)n-1 + H2O = M+(H2O)n,Īn Experimental and Ab Initio Study of the Nature of the Binding in Gas-Phase Complexes of Sodium Ions,Ĭhem. Hydration of the Alkali Ions in the Gas Phase. Binding Energies of Li+ to pi - and n - Donor Bases, Intrinsic Acid - Base Properties of Molecules. Thermochemistry of Organic and Organometallic Compounds, Academic Press, New York, 1970, 1-636. Heats of formation of liquid methyl sulfoxide and crystalline methyl sulfone at 18°, A reevaluation of the upper proton affinity range, Proton affinity ladders from variable-temperature equilibrium measurements. Peschke, M.,Ī Definitive Investigation of the Gas-Phase Two-Center Three-electron Bond in, +, and +: Therory and Experiment, Mutual Effects of Weak and Strong Ligands in Mixed Clusters,ĭeng, Y. The Ionic Hydrogen Bond and Ion Solvation. Gas phase chemistry of alpha-thio carbanions,Ĭan. Photoelectron Spectroscopy of Sulfur Ions, Go To: Top, Reaction thermochemistry data, Notes
#Dimethyl sulfide charge free#

Gas phase switching reaction(Li+)H2O, from graph Dzidic and Kebarle, 1970 extrapolated M Liquid phase Reanalyzed by Cox and Pilcher, 1970, Original value = -278.3 ± 0.8 kJ/mol At 291°K ALS Gas phase Δ rH?, inconsistent with other protonated sulfur dimers Mīy formula: C 2H 6S + + C 2H 6S = ( C 2H 6S + īond type: Charge transfer bond (positive ion)īy formula: C 4H 9 + + C 2H 6S = ( C 4H 9 + īy formula: 2 C 2H 6S + O 2 = 2 C 2H 6OS Quantity Reaction search pages in place of the enumerated reactionīy formula: C 2H 5S - + H + = C 2H 6S Quantityīy formula: C 2H 7S + + C 2H 6S = ( C 2H 7S + Secretary of Commerce on behalf of the U.S.A. Your institution may already be a subscriber.įollow the links above to find out more about the dataīy the U.S. With the development of data collections included in The purpose of the fee is to recover costs associated NIST subscription sites provide data under theĭata Program, but require an annual fee to access.
#Dimethyl sulfide charge professional#
NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data).NIST / TRC Web Thermo Tables, "lite" edition (thermophysical and thermochemical data).X-ray Photoelectron Spectroscopy Database, version 4.1.Computational Chemistry Comparison and Benchmark Database.Use this link for bookmarking this species This structure is also available as a 2d Mol file IUPAC Standard InChIKey: QMMFVYPAHWMCMS-UHFFFAOYSA-N Copy.


 0 kommentar(er)
0 kommentar(er)
